
About AccuMix Pharmacy
AccuMix Pharmacy is a 503A sterile and non-sterile compounding pharmacy dedicated to one thing: delivering safe, precise, patient-specific medications that meet your prescriber’s exact directions. From medical weight loss to HRT, peptides, IV wellness, dermatology, sexual health, and allergy immunotherapy, our team crafts every preparation for purity, potency, and consistency.
WHO
WE
ARE?
We’re a pharmacist-led organization with Board-Certified Sterile Compounding Pharmacists (BCSCP) on staff, supported by highly trained technicians and a culture of continuous quality improvement. Our team blends clinical expertise, USP <795>/<797>/<800> standards, and rigorous QA to deliver precise, patient-specific sterile and non-sterile preparations in close collaboration with prescribers.
Our facility
&
cleanrooms
Compounding happens in a cGMP-aligned environment with documented traceability, environmental monitoring, and multi-level quality checks. We maintain ISO Class 7 cleanrooms with ISO Class 5 laminar airflow hoods, HEPA filtration, segregated hazardous/non-hazardous workflows, and 24/7 temperature/humidity controls—fully compliant with USP <795>, <797>, and <800>.
We perform media fills, endotoxin screening, and routine environmental monitoring under FDA/USP guidance to safeguard sterility and consistency from formulation through final pharmacist release.
What we compound
AccuMix serves modern wellness and specialty needs with individualized dosing and delivery methods
Advanced compounded options prepared in sterile, USP-compliant conditions with strict QC for consistency, purity, and stability.
Bioidentical formulations (topical, injectable, troche) compounded to lab data and provider protocols; DEA-licensed for controlled preparations where appropriate.
Regenerative and performance-oriented compounds (e.g., BPC-157, CJC/Ipamorelin, NAD⁺, glutathione) produced under USP standards with sterility/potency verification.
Men’s and women’s therapies compounded to support function, arousal, and performance under strict USP guidelines.
Custom sterile IV blends (e.g., Myers’, NAD⁺, Immune, Performance) prepared in ISO Class 5 hoods within an ISO Class 7 cleanroom per USP <797>.


Quality, safety & compliance
Our operating system is built for reliability: cGMP-aligned protocols, full lot traceability, FDA-registered sourcing with COA verification, and multi-step QA reviews before pharmacist release. We align to FDA, DEA, and state board regulations for compounding, storage, and dispensing, and we maintain continuous training against current Good Compounding Practices.
Important FDA note: Compounded medications are prepared under federal and state regulations but are not reviewed or approved by the FDA. We source from FDA-registered suppliers and follow FDA/USP guidance for sterility assurance.
DEA compliance: AccuMix is DEA-licensed, enabling appropriate handling and dispensing of controlled preparations within the scope of prescriber orders and state rules.
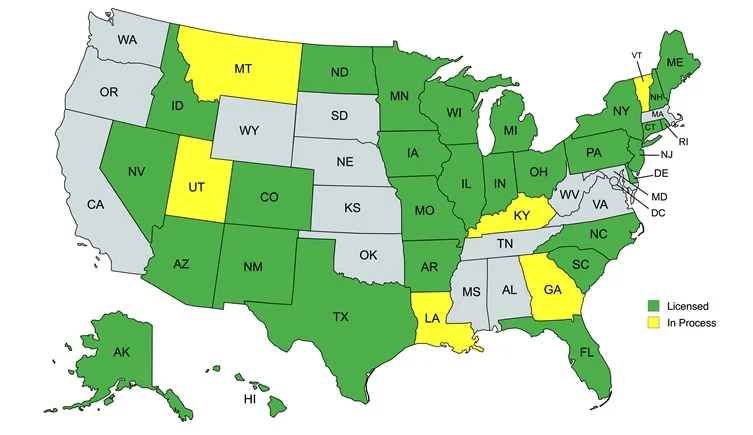
Licensing & Nationwide Reach
Headquartered in Elmhurst, Illinois, AccuMix maintains active state board of pharmacy licenses across multiple U.S. states, allowing us to compound and dispense sterile and non-sterile medications to patients and providers nationwide while adhering to each jurisdiction’s rules. (Want to display a state-by-state list? We can place it here as a collapsible panel.)
All operations remain in compliance with DEA and FDA requirements for sterile and non-sterile compounding activities alongside state regulations

Who
we serve
Patients
One-to-one care, counseling, and dosing plans coordinated with your provider—because your health isn’t one-size-fits-all.
Our process
(from prescription to delivery)
Team credentials & oversight
AccuMix employs pharmacists with advanced training in sterile technique and BCSCP certification, supported by technicians trained in aseptic practice, documentation, and environmental controls. Ongoing audits, training refreshers, and performance metrics reinforce a culture of safety and continuous improvement.



Sourcing
&
materials
We use pharmaceutical-grade APIs from FDA-registered suppliers; every lot is verified with a Certificate of Analysis (COA) before release to compounding.
For providers & telehealth partners
We offer collaborative formulary development; documentation and titration protocols (e.g., allergy immunotherapy kits and SLIT programs); and sterile IV, hormone, peptide, and dermatology compounds harmonized to your protocols. Our team provides pharmacist consults and onboarding support to operationalize multi-site or multi-state rollouts with consistent QA.
Compliance & disclosures
AccuMix operates as a 503A compounding pharmacy; preparations are made only after receipt of a valid patient-specific prescription. Compounded medications do not undergo FDA approval but are prepared under federal/state regulations and USP standards; we follow FDA/USP guidance for sterility and quality assurance and maintain DEA licensure where applicable.


